Sterilization and Preheating Difference
For biological and pharmaceutical dosage forms sterilization is required. sterilization is most important to prevent the external contamination of viable organisms which is to prevent microbial growth inside the dosage forms. There are many types of sterilization and are mentioned below. Microbial contamination is very dangerous, which can cause patient death. So this post prepared, to understand Sterilization and preheating differences.
Types of Sterilization:
- Moist
heat sterilization
- Radiation
sterilization
- Dry
heat sterilization
- Gas
sterilization
- Chemical
sterilization.
- Filteration
Though the temperature is a determining factor for sterilization, there is a difference between sterilization temperature and preheating temperature.
What is a sterilization temperature?
In normal cases, sterilization temperature is a temperature at which the microorganism present in the load are killed. Normally sterilization temperature is different for various methods of sterilization. For moist heat sterilizers, 121°C for 15 LBS pressure is a requirement. For dry heat sterilizers, more than 300°C are required for Depyrogenation. For heat-sensitive products again the temperature is reduced and exposure time is increased.
Sponsored Search:
What is preheating temperature?
However, preheating is required to achieve sterilization temperature. We cannot operate the autoclave which has temperature more. Hence during the load preparation phase, the temperature of the autoclave / or any sterilizer is cooled down. Again after loading articles for sterilization, the sterilizer will start to heat up to gain temperature. This is called as preheating.
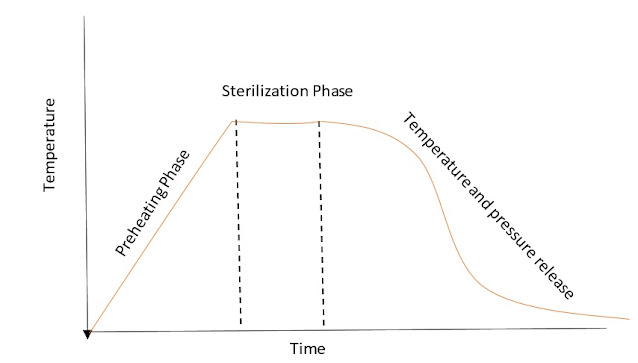
See the below graph that will differentiate between sterilization temperature and preheating temperature. For sterilization to occur preheating temperature is a must. Then only it will raise to sterilization temperature. All the sterilizers are controlled through software, so as per the set program, the required temperature will raise automatically. It has the controls to stop the temperature also.
👌👌
ReplyDeleteThank you Prashant sir for the elaborated explanation.
ReplyDeleteYarsons International is committed to manufacture better products with advanced technology.
ReplyDelete