Aseptic manufacturing and terminal sterilization difference - Basics of aseptic processing
This post is related to pharmaceutical sterile drug manufacturers. So many sterile dosage forms are there and all of them are either terminally sterilized or aseptically manufactured. There is a huge difference between aseptic manufacturing and terminal sterilization. Both these are the sterile process.
Aseptic manufacturing is more critical if compared with terminal sterilization. As the sterility failure chances are more in the aseptically manufactured products. Hence basics of aseptic manufacturing are required to know.
What is aseptic manufacturing?
The sterilized product or components are brought together and are assembled in a controlled environment. This is called aseptic manufacturing.In this process, sterility is been ensured by aseptic connection, filtration through a 0.22-micron filter. Also, to maintain a clean area, pharmaceutical isolators, isolator gloves systems, and barrier systems are currently utilized.
But this process is tedious compared to terminal sterilization. For all the sterilization processes autoclave plays an important role. Hence, the autoclave is called the "heart of the sterile manufacturing industry".
In this process, full and final filled products will undergo sterilization. As it undergoes final sterilization, any chance of microbial contamination will be removed.
Why aseptic manufacturing is being done? What are the basics of aseptic manufacturing?
- For those products which cannot terminally be sterilized for them, the aseptic manufacturing process is must be required.
- For the manufacturing of eye drops, injections, and respules aseptic manufacturing process is utilized.
- For the respules and eye drops, the primary packing components are HDPE bottles which are heat sensitive. Hence terminal sterilization method can not be utilized.
- However, many parenteral products are filled in glass vials. But due to the heat liability of filled products, terminal sterilization can not be done.
- Rest all other parenteral products can undergo terminal sterilization.
Recent posts
1. HEPA filter classification for sterile manufacturing unit and associated questions – By Prashant Devmore2. What is HVAC (AHU) system? How AHU operates? - By Prashant Devmore
3. Dissolution & Related Technical Questions – By Bhagyashree Chothe
4. How to check broken links of website in 2019? - By Prashant Devmore
What are the controls in the aseptic manufacturing process:
- As I said, aseptic manufacturing involves assembling sterilized articles in a controlled environment, the false-positive results may occur because of internal testing methodology.
- Sterility is a prime consideration for releasing sterile product in the market.
- There should be strict control of cleanroom practices.
- Cleanroom procedures must be well developed to arrest contamination.
- To maintain the cleanroom area, the HVAC system should have temperature and humidity control.
- The sterile manufacturing process and equipment must be well designed to arrest foreign particles.
- The area gradation must be monitored periodically, to ensure the class of the area.
- Accessories required for usage must be prior sterilized.
- The autoclave validation process shall be developed and shall be validated periodically.
 |
| Get 70 % Off on Home Decor |
Limitations of Sterile manufacturing process-
- Cleanroom design, procedures, and practices shall be well developed.
- Pressurized articles or ready-to-use articles are required. Which increases processing costs.
- Increased chances of bioburden as man movement increases.
- Sterility assurance level (SAL) must require for all aseptic processing units.
- Building and facility should be well enough to arrest foreign particles inside the area.
- To maintain class gradation, laminarity of air, the HVAC system must be in place 24/7
- Premises, walls, the floor shall be smooth and shall resist microbial growth.
- At every stage of processing, hand sanitization is must require to kill the bioload present on gloved hands.
- Disinfectant efficacy should be validated. An alternate disinfectant is required to reduce the tolerance level of microorganisms. Personnel entering the critical areas should be qualified for the evaluation of microbial load.
- The entry/ exit procedure must be well defined to reduce the incoming of foreign particles inside the area.
- Any intervention, manipulation, processing of activities during assembling aseptically pocess a major risk to product quality. Hence the same to be controlled.
- Annually each manufacturing process shall be simulated for media fill studies. Media fill studies are the most important criteria for sterile manufacturing processes.
- Each critical area has to be monitored for the environmental viable and non-viable count. Data of the same is required to be trended monthly, quarterly, and annually to evaluate the microbial excursions.
- Environmental isolates identification is required to identify the source of the microorganisms.
- Sterility testing is required in critical areas to minimize false-positive results. For overcoming these false-positive results issues isolator technologies can be utilized.
 |
| Offer: Buy Women's Footwear Sports Shoes Starting From At Rs.237/- |
- Sterility testing isolators-
These are required to reduce false sterility test failures. This consists of three modules or cubicles generally one for sterilization of accessories, 2nd for sterility testing, and third for material transfer. Usually, it involves glove ports to minimize the manual interventions inside the cubicle
A detailed post on sterility test isolator is at the below link:
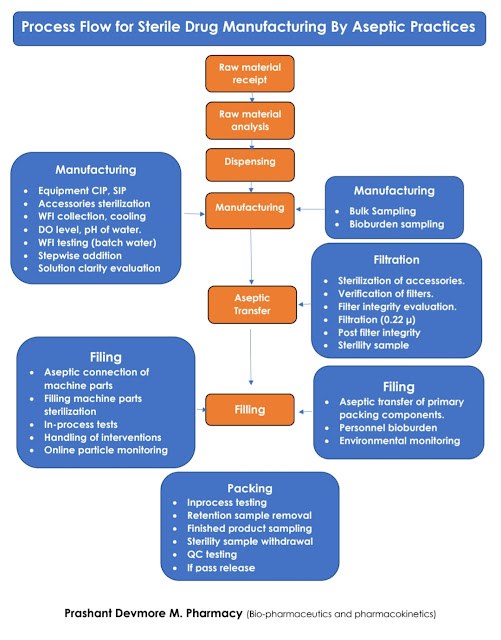
Sterile Manufacturing Process Flow
1. Aseptic Manipulations
2. Terminal sterilization:
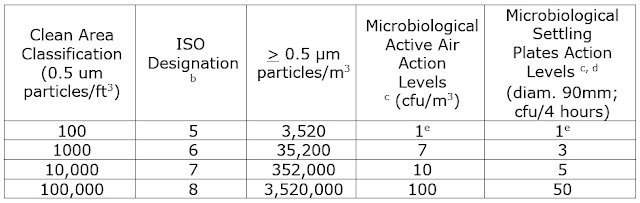
Clean Area Classification
Reference: Guidance for Industry https://www.fda.gov/media/71026/download
TABLE 1- Air Classificationsa
a- All classifications based on data measured in the
vicinity of exposed materials/articles during periods of activity.
b- ISO 14644-1 designation provide uniform particle
concentration values for clean rooms in multiple industries. An ISO 5 particle concentration is equal to
Class 100 and approximately equals EU Grade A.
c- Values represent recommended levels of environmental
quality. You may find it appropriate to
establish alternate microbiological action levels due to the nature of the
operation or method of analysis.
d- The
additional use of settling plates is optional.
e- Samples from
Class 100 (ISO 5) environments should normally yield no microbiological
contaminants.
Readers also search for -
Sterilization | Sterility Assurance Level
Difference Between Aseptic and Terminal Sterilization
Quality Assurance Interview Questions In Pharma Industry
 |
| Offer : Buy Smart Tvs Starting From At Rs.9990/- |


I live in Madagascar ( East Africa),and life is worth living comfortably for me and my family now and really have never seen goodness shown to me this much in my life as I have been going through a problem as seriously as my son found a terrible accident last two weeks, and the doctors states that he needs to undergo a delicate surgery for him to be able to walk again and I could not pay the bills, then your surgery went to the bank to borrow and reject me saying that I have no credit card, from there i run to my father and he was not able to help, then when I was browsing through yahoo answers and i came across a loan lender Mr, Benjamin Breil Lee, offering loans at affordable interest rate I had no choice but to give it an attempt and surprisingly it was all like a dream, I got a loan of $ 110,000.00 to paid for my son surgery then get myself a comfortable business to help me going as well. I thank God today is good and you can walk and is working and the burden is longer so much on me more and we can feed well and my family is happy today and i said to myself that I will mourn aloud in the world of the wonders of God to me through this God fearing lender Mr Benjamin Breil Lee and I would advise anyone in genuine and serious need of loan to contact this God-fearing man on ...... 247officedept@gmail.com through .. and I want you all to pray for this man for me or Chat with him on whatsapp +1-989-394-3740 as well.
ReplyDeleteThank you