Difference Between Aseptic and Terminal Sterilization
In a pharmaceutical company, the drug products are manufactured. For parenteral, ophthalmic, and nasal drug products sterility of product till the end of shelf life is the biggest concern. All these drug products are directly affecting the body if aseptic condition and product sterility is not maintained.
Hence, the parenteral products and ophthalmic products are sterilized in different ways such as steam sterilization, terminal sterilization, dry heat sterilization, gamma irradiation, and filtration. However, at all the stages aseptic processing matters a lot. Hence to understand the difference between aseptic and terminal sterilization, this post is written.
In view of controlling microorganisms' growth, the drug products are sterilized. There are different ways of sterilization. Initially, the product's contact parts are pre-sterilized and assembled aseptically and at the final stage, the terminal sterilization is being carried out for a full product-filled container. Aseptic manipulations can control the growth of microorganisms. But to increase sterility assurance level terminal sterilization is the effective way. However terminal sterilization has few limitations to heat liable products.
Now in detail difference between aseptic and terminal sterilization is mentioned below.
Read more on Autoclave
Aseptic activity:
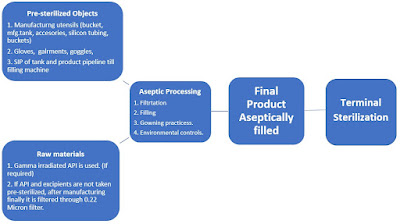
First of all, clear that aseptic processing is not a sterilization method. It is one type of contamination control methodology in cleanroom areas.
In this process, the sterilized, cleaning accessories are brought together maintaining cleanroom conditions, behavioral practices, and good aseptic practices and assembled to have a final product. At each stage, contamination risk is considered and procedures are set to manufacture a product.
Terminal sterilization:
Terminal sterilization is the process of sterilizing a product in its final container. It is an important process as it ensures the product remains sterile. All medical, ophthalmic, and parenteral equipment are sterilized in batches, and usually sterilized using heat. However, this method has few limitations of heat. For the products which are heat liable, those products are only pre-sterilized and aseptically filled.
Sterilization
Sterilization can be defined as any process that effectively kills or eliminates microorganisms and transmissible agents (such as fungi, bacteria, viruses) from surfaces, equipment, foods, medications, and from the biological culture mediums.
A sterility assurance level (SAL) of 10-6 means that there is less than or equal to one chance in millions that an item is contaminated or unsterile following the sterilization process.
The autoclave was invented by Charles Chamber land in 1879. The name comes from Greek auto-self, and Latin clevis- key, a self-locking device.
Types of Sterilization
1. Moist heat sterilization
Typically, moist heat sterilization (or autoclaving or steam sterilization) is used in pharmaceuticals, hospitals for sterilizing the surfaces of various utensils, including hollow items or wrapped goods. This process is performed by supplying dry, saturated steam under pressure into an autoclave. Steam quality plays a great role in steam sterilization. Steam Quality Test. The steam sterilization temperature requirement is mentioned below.
Sr. no. | Temperature (℃) | Pressure(psi) | Time |
1 | 115℃ | 10 psi | 30 minutes |
2 | 121℃ | 15 psi | 15 minutes |
3 | 132℃ | 27 psi | 10 minutes |
2. Dry heat sterilization
The dry heat sterilization process is performed by conduction; that is where heat is absorbed by the exterior surface of an item and then passed onward to the next layer. Eventually, the entire item reaches the proper temperature needed to achieve sterilization. The proper time and temperature for dry heat sterilization is 160 °C (320 °F) for 2 hours or 170 °C (340 °F) for 1 hour or in the case of High-Velocity Hot Air sterilizers 190°C (375°F) for 6 to 12 minutes.
3. Chemical sterilization
Chemical Sterilization is the process of removal of microorganisms by the use of chemical bactericidal agents E.g. H2O2 disinfection.
4. Gaseous sterilization
Sterilizing gases are typically used when exposure to other methods (heat or radiation) could damage the materials or equipment. The most common gases used for sterilization include ethylene oxide (EO), ozone, mixed oxides of nitrogen, and chlorine dioxide.
E.g. eto sterilization
5. Filtration
Filtration is common for liquid products that are manufactured in the pharmaceutical. The filtration not only removes the microbes but also removed particulate matter present in the formulation. Only the extent of filtration level is important. For this 0.22 Micron size filter is commonly used to remove microorganisms.

Comments
Post a Comment
If you have any doubt please let me know. Thank you.