Equipment Qualification | Equipment Qualification Flowchart
Abstract
The pharmaceutical industry is full of equipment. Using this equipment only medicines, vaccines, lifesaving drugs are getting manufactured. Hence, in this post detailed review of equipment qualification is provided along with an equipment qualification flowchart.
Qualification is a state which comes 1st in pharmaceutical manufacturing industry development. Qualification generally refers to each criterion including process qualification, product qualification, personnel qualification, equipment qualification, area qualification, HVAC qualification.
It is a state of validating the object for routine use. It generally comes with the terminology of validation. Validation is nothing but a qualification. In this post, I will elaborate only on the equipment qualification process.
Validation:
The term qualification starts with industry development. So write from the user requirements till the release of equipment, the qualification term will remain. After a certain period, periodic requalification is also required. We will go one by one.
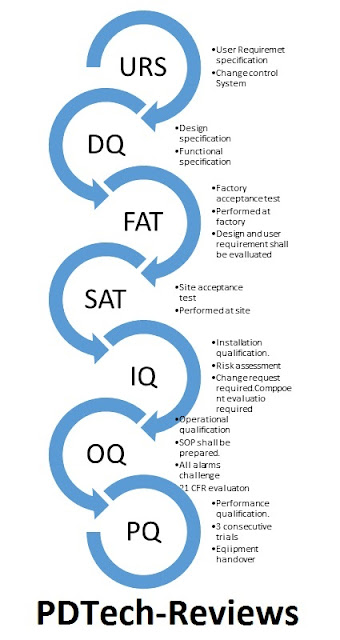
What is IQ OQ and PQ in pharmaceutical equipment qualification? What is IQ OQ PQ DQ in pharma?

DQ stands for design qualification in
pharma. In this stage the design of the equipment is evaluated for ensuring user requirements have been considered while designing the equipment. This stage of qualification comes after the finalization of user requirements specification. And after this stage installation can be proceeded if FAT and SAT are completed. For ready-to-use equipment’s FAT and SAT requirements are not mandatory.
IQ stands for installation qualification. This is the stage that demonstrates the installation of equipment at the site. Along with the vendor/service engineer the already designed equipment is assembled at the installation site. The installation procedure shall be documented and that document is referred to as an installation qualification document.

OQ stands for operational qualification. This stage comes after the installation of equipment is completed and certified by the vendor. In this stage of qualification, the equipment operation is checked against the operating requirement that was provided through user requirement specification.
Note: This stage includes (But is not limited to) the following,
Also, in this stage only, the standard operating SOP shall be prepared and SOP shall be available for execution of performance qualification.
PQ stands for performance qualification. This stage comes after the successful execution of the operational qualification of equipment. This stage demonstrates that, the equipment operation with respect to all features including, machine parts, sensors, alarms, safety features, calibration, display panels, HMI, PLC, SCADA parameters are operational as per pre-defined requirements. Hence only the performance of that equipment is to be evaluated through this stage. In this stage, the equipment is run for continuous operation and for three consecutive runs. IF all the three consecutive runs of equipment qualification are satisfactory, then equipment can be qualified for routine use.
Steps of equipment qualification? What is the sequence of pharmaceutical equipment qualification?
To perform the qualification of equipment, the equipment has to undergo many challenges. These challenges are pre-defined and will be executed stage-wise. The stages of qualification of equipment are mentioned below. The process starts with the change control system.
Read the full article to go deeper into the pharmaceutical equipment qualification.
Why pharmaceutical equipment qualification is required?
As we know, medicines are used to treat human lives or living organisms, the manufacturing process of medicines is very critical. Each step of the medicine manufacturing process is crucially monitored and evaluated. Based on the satisfactory release of all test parameters only the medicines are getting released. However, at each stage, there are different types of machines i.e. manufacturing tanks, pressure vessels, autoclaves, tunnels, filling machines, packing machinery, Checkweigher, laboratory test equipment, etc. All these equipment’s before use are need to qualify to ensure, the procures machine is operating and performing as intended.
What is pharmaceutical equipment qualification?
equipment Qualification is the process in which the equipment operation and performance are evaluated and documented to ensure that, the equipment, fulfills the operation and performance requirement at the site.
People also search for:
What makes equipment qualification successful? What is equipment qualification in pharma?
1. Successful installation of equipment.2. Successful installation of equipment parts like computer, PLC, HMI, hardware components, instrument, sensors and software systems.3. Evaluation of standards.4. Evaluation of calibration details of instrument5. Successful operation of equipment.6. Availability of standard operating procedure.7. Successful alarm challenges.8. Successful audit trail execution.9. Successful computerized system challenges.10. Successful 3 consecutive machine performance challenges.
What is performance qualification of pharmaceutical equipment?
After successful comption of operational qualification, the equipment has to undergo performance qualification. In this stage, the equipment to be challenged to evaluate the performance of equipment and ensure the objectives for which the equipment is procured is fulfilled. As per guidance review, for performance qualification, 3 consecutive runs is must required. 1st successful run shall be considered as "accidental results". 2nd successful run shall be considered as "coincidence", and 3rd successful run shall be considered as confirmed results. after these three consecutive successful runs, the equipment shall be released for routine usage.





Nice ...
ReplyDeleteGood Writing . Please write more in details. I guess the V chart for Equipment Qualification more helpful than the Flow you have given. The V chart indicates the responsibilities of Vendor and User . In case of new machine in new premises the PQ is very challenging. We can't run commercial batches , so in that case Process Validation and Equipment performance Qualification can be done together. Sometimes OQ and PQ can be conducted together.
ReplyDeleteThanks for writing up
Best Regards